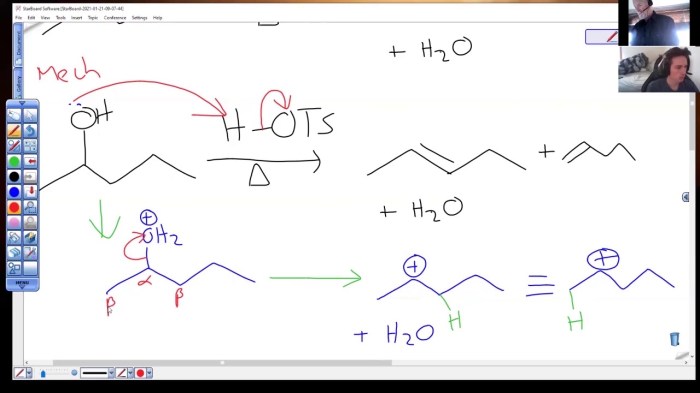
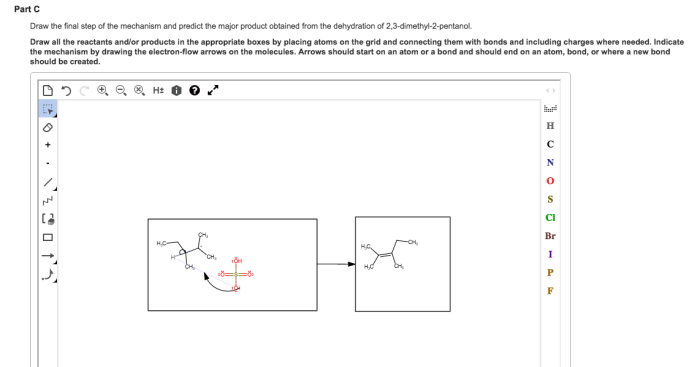
Draw the major product for the dehydration of 2-pentanol. – In the realm of organic chemistry, the dehydration of 2-pentanol stands as a captivating transformation, unveiling the intricacies of regioselectivity, stereochemistry, and industrial applications. This comprehensive guide delves into the depths of this reaction, unraveling its mechanisms, factors influencing its course, and practical implications.
The dehydration of 2-pentanol, a process of removing water to form an alkene, offers a glimpse into the power of acid catalysis and the delicate balance of regio- and stereoselectivity. By examining the interplay of these factors, we gain insights into the precise control of product formation, paving the way for targeted synthesis of valuable organic compounds.
Dehydration of 2-pentanol
Dehydration is a chemical process that involves the removal of water molecules from a compound. In organic chemistry, dehydration reactions are commonly used to convert alcohols into alkenes.
The dehydration of 2-pentanol is a classic example of an alcohol dehydration reaction. The reaction proceeds via an E2 elimination mechanism, which involves the simultaneous removal of a proton from the β-carbon and a hydroxyl group from the α-carbon.
Mechanism of the Dehydration of 2-pentanol
The dehydration of 2-pentanol occurs in the presence of an acid catalyst, such as sulfuric acid or phosphoric acid. The mechanism of the reaction is as follows:
- The acid catalyst protonates the hydroxyl group of 2-pentanol, forming a protonated alcohol.
- The protonated alcohol undergoes an E2 elimination reaction, resulting in the formation of an alkene and water.
- The alkene product is typically a mixture of E and Z isomers.
Regioselectivity in the Dehydration of 2-pentanol, Draw the major product for the dehydration of 2-pentanol.
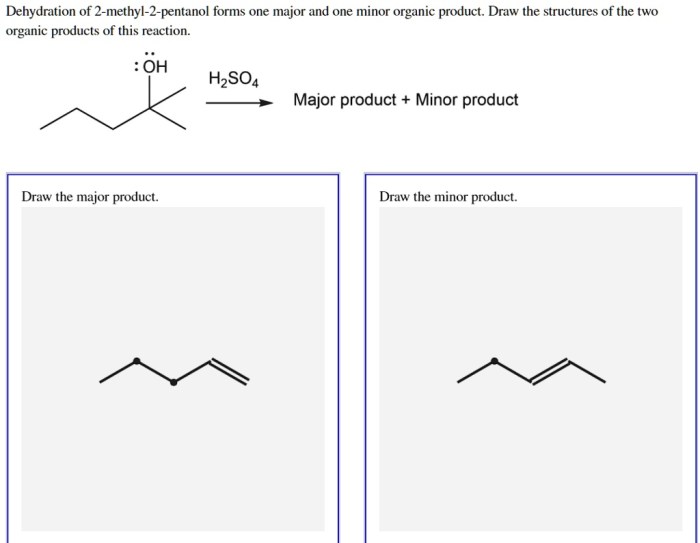
Regioselectivity refers to the preference for one reaction pathway over another. In the dehydration of 2-pentanol, there are two possible products: 2-pentene and 3-pentene.
The regioselectivity of the dehydration reaction is determined by the stability of the carbocation intermediate that is formed during the reaction. The more stable the carbocation, the more likely it is to be formed, and the more likely the corresponding alkene product will be formed.
In the dehydration of 2-pentanol, the formation of 2-pentene is favored over the formation of 3-pentene because the carbocation intermediate that is formed during the formation of 2-pentene is more stable than the carbocation intermediate that is formed during the formation of 3-pentene.
Stereochemistry of the Dehydration of 2-pentanol
The dehydration of 2-pentanol can produce both E and Z isomers of the alkene product. The stereochemistry of the alkene product is determined by the orientation of the hydrogen and alkyl groups on the β-carbon relative to each other.
In the dehydration of 2-pentanol, the E isomer is typically the major product. This is because the E isomer is more stable than the Z isomer due to less steric hindrance between the hydrogen and alkyl groups on the β-carbon.
Applications of the Dehydration of 2-pentanol
The dehydration of 2-pentanol is a versatile reaction that has a wide range of applications in both industry and the laboratory.
- In industry, the dehydration of 2-pentanol is used to produce 2-pentene, which is a valuable starting material for the synthesis of a variety of other organic compounds, such as plastics, solvents, and fuels.
- In the laboratory, the dehydration of 2-pentanol is used as a model reaction for studying the mechanism of E2 elimination reactions.
Clarifying Questions: Draw The Major Product For The Dehydration Of 2-pentanol.
What is the major product of the dehydration of 2-pentanol?
The major product is 2-pentene, formed via a Zaitsev’s rule-favored E2 elimination.
What factors influence the regioselectivity of the dehydration of 2-pentanol?
The regioselectivity is influenced by the stability of the carbocation formed during the reaction, with the more substituted carbocation being more stable and favored.
How can the stereochemistry of the dehydration of 2-pentanol be controlled?
The stereochemistry can be controlled by using specific catalysts or reaction conditions that favor the formation of either the E or Z isomer.