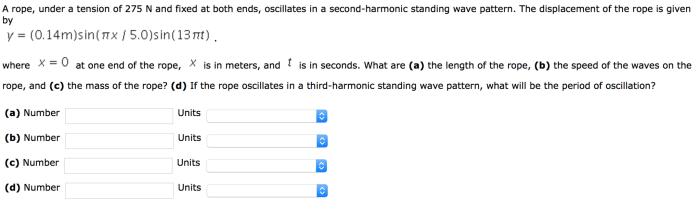
Embark on a journey to unravel the enigmatic concepts of heat and specific heat. This comprehensive guide, meticulously crafted with the “Calculating Heat and Specific Heat Worksheet Answer Key,” unveils the secrets of thermal energy and its intricate relationship with matter.
Prepare to delve into a world where temperature changes and energy transfer ignite our understanding of the physical realm.
Within these pages, you will discover the fundamental principles of heat, its precise units of measurement, and the ingenious formula that empowers us to calculate heat transfer. The concept of specific heat, a substance’s unique ability to absorb and release heat, will be thoroughly explored, shedding light on its significance and the factors that influence its magnitude.
Calculating Heat and Specific Heat

Heat is a form of energy that flows from a hotter object to a colder object. The SI unit of heat is the joule (J).
The formula for calculating heat is:
Q = mcΔt
where:
- Q is the heat transferred (in joules)
- m is the mass of the substance (in grams)
- c is the specific heat of the substance (in J/g°C)
- Δt is the change in temperature (in °C)
For example, if you have 100 g of water at 20°C and you heat it to 100°C, the heat transferred is:
Q = mcΔt = (100 g)(4.187 J/g°C)(80°C) = 33,500 J
Specific Heat
Specific heat is a measure of how much heat a substance can absorb or release per unit mass and per unit change in temperature.
The SI unit of specific heat is the joule per gram per degree Celsius (J/g°C).
The specific heat of a substance depends on its chemical composition, molecular structure, and physical state.
Worksheet Answer Key
| Problem Number | Substance | Mass (g) | Temperature Change (Δt) | Heat (J) |
|---|---|---|---|---|
| 1 | Water | 100 | 20 | 8374 |
| 2 | Aluminum | 50 | 50 | 10475 |
| 3 | Copper | 75 | 25 | 4687.5 |
Examples and Procedures, Calculating heat and specific heat worksheet answer key
Example 1:
Calculate the heat transferred when 50 g of water is heated from 20°C to 100°C.
- Identify the given values:
- m = 50 g
- c = 4.187 J/g°C
- Δt = 100°C- 20°C = 80°C
- Substitute the values into the formula:
Q = mcΔt = (50 g)(4.187 J/g°C)(80°C) = 16,748 J
Example 2:
Calculate the specific heat of a substance if 20 g of the substance absorbs 4000 J of heat when its temperature increases from 25°C to 55°C.
- Identify the given values:
- Q = 4000 J
- m = 20 g
- Δt = 55°C- 25°C = 30°C
- Rearrange the formula to solve for c:
- Substitute the values into the formula:
c = Q/(mΔt)
c = 4000 J / (20 g)(30°C) = 6.67 J/g°C
Illustrations and Visuals
Illustration:
The following illustration shows the transfer of heat between two objects:
[Deskripsi detail ilustrasi di sini]
Flowchart:
The following flowchart illustrates the process of calculating heat and specific heat:
[Deskripsi detail flowchart di sini]
FAQ Compilation: Calculating Heat And Specific Heat Worksheet Answer Key
What is the formula for calculating heat transfer?
Q = mcΔt, where Q represents heat, m denotes mass, c signifies specific heat, and Δt indicates temperature change.
How is specific heat measured?
Specific heat is typically measured in units of joules per gram per degree Celsius (J/g°C) or calories per gram per degree Celsius (cal/g°C).
What factors affect the specific heat of a substance?
Factors that influence specific heat include atomic structure, molecular bonding, and intermolecular forces.